Past Issues
The Antimicrobial Property of Plasma Activated Liquids (PALs) against Oral Bacteria Streptococcus mutans
Qing Hong1*, Xiaoqing Dong2, Haiqing Yu3, Hongming Sun3, Meng Chen2, Yong Wang4, Qingsong Yu1*
1Department of Mechanical and Aerospace Engineering, University of Missouri, Columbia, MO 65211, USA
2Nanova, Inc., Columbia, MO 65202, USA
3Department of Internal Medicine, University of Missouri, Columbia, MO 65211, USA
4Center for Research on Interfacial Structure & Properties, School of Dentistry, University of Missouri-Kansas City, Kansas City, MO 64108, USA
*Corresponding author: Dr. Qingsong Yu, Department of Mechanical and Aerospace Engineering, University of Missouri, Columbia, MO 65211, USA; E-mail: [email protected]
Received Date: December 03, 2020
Publication Date: May 30, 2021
ABSTRACT
Objective: The ability of plasma activated liquids (PALs) to disinfect oral bacteria in both planktonic and biofilm forms were investigated.
Material and Methods: Phosphate-buffered saline (PBS) and 0.9% saline liquids were activated by non-thermal atmospheric pressure plasma treatment to produce antimicrobial PALs. These PALs were then used to culture oral bacteria Streptococcus mutans (S. mutans) in both planktonic and biofilm forms. The antimicrobial efficacy was assessed by MTT (3-4,5-Dimethylthiazol-2,5-Diphenyltetrazolium Bromide) and colony forming unit (CFU) assays. The data was evaluated by One-way ANOVA statistical analysis.
Results: The PALs could inactivate 38% of the planktonic bacteria and induce 25% biofilm reduction. It was further found that the PALs aged for 24 hours were not effective on bacterial disinfection. However, PALs were found to be non-toxic for mammalian cells. No viability change of fibroblast cells was detected in PALs. Hydrogen peroxide content increased in PALs than that in untreated liquids.
Conclusion: The PALs showed instant antimicrobial effects on oral bacteria of S. mutans in both planktonic and biofilm forms. However, PALs could not completely disinfect all the bacteria and were not able to inhibit the bacteria recovery. It was also found that the PALs were non-toxic to mammalian cells.
KEYWORDS: Dental biofilms; Plasma Activated Liquids; Plasma-liquid Interactions; Antimicrobial
INTRODUCTION
Non-thermal plasma treatment has been widely considered as an effective method for sterilization. According to Koban I, et al.[1], application of plasma treatment was much more effective than chlorhexidine digluconate (CHX) in disinfecting biofilms. In a study by Xiong Z, et al. [2], plasma was found to penetrate biofilms and effectively deactivated all the bacteria in 15μm thick biofilms. Another study by Rupf et al. also confirmed the disinfection capability of plasma treatment for dental biofilms [3-5]. Non-thermal atmospheric plasma could deactivate gram positive bacteria (S. mutans)[6], gram negative bacteria (P. gingivalis)[2] and fugus (C. albicans)[7] in the form of biofilms.
Even though it is effective in bacterial sterilization tool, direct plasma treatment is a little challenging to delicate surfaces and tissues [8]. Direct plasma application may cause slight yet measurable tissue damage due to the high energy electrons and ultraviolet (UV) photons generated during plasma excitation [8,9].
Plasma tissue damage has motivated research into indirect plasma treatments. One type of indirect plasma application is plasma coatings on target material. These coating plasmas are created in vacuum chambers under low pressure conditions [7].Plasma-deposited coatings using silane () and nitrous oxide as precursor gases also showed some effectiveness in reducing bacterial adhesion to the dental material surface [8].
Another indirect application of plasmas [9-11] is treating liquids to generate antibacterial species in the liquids. There is evidence that bacteria can be killed by contact with liquids that had first been activated by non-thermal plasmas. This kind of plasma treated liquids with disinfection capability is called plasma-activated liquids (PALs). PALs can be considered as a convenient disinfection solution by overcoming the lack of portability of the plasma systems. A study by Ercan UK, et al. [9] demonstrated plasma-activated PBSgave rapid inactivation of various pathogens. Kamgang-Youbi G, et al.[10] also showed the efficacy of plasma activated water on disinfecting planktonic bacteria and biofilms. In addition, PALs are also beneficial to the treatment of contaminated water. Du CM, et al. [11] developed a gliding arc discharge reactor to treat E. coli contaminated water. Almost 100% of the bacteria were killed in less than 3 min when the contaminated water was passed through the plasma reactor and circulated at defined flow rates [11].
The study of reactions between plasma and liquid is an important topic since plasma comes in direct contact with biological fluids in most biomedical applications [12]. However, the exact mechanism and contribution of various plasma species to the biological effect are not yet fully understood. This is due to the complex interactions [13] at the plasma-liquid interface and subsequent reactions in the liquid phase. Among various chemical species produced by plasma at the gas-liquid interface, OH· radicals, atomic oxygen, ozone and hydrogen peroxide are the main reactive oxygen species (ROS) generally accepted to play dominant roles in the inactivation process [14]. Contribution of nitrogen-based reactive species (RNS) must also be considered. Examples of RNS include nitric oxide and its derivatives formed within water, including nitrites, nitrates, and peroxynitrites [15,16].
The aim of this study is to assess the ability of plasma activated liquids (PALs) in disinfecting oral bacteria in both planktonic and biofilm forms. PBS and 0.9% saline were activated by the non-thermal atmospheric plasma, and their antimicrobial properties were tested on S.mutans in both planktonic and biofilm states. The H2O2 concentration in the PAL was assessed in order to understand the mechanism of PAL inactivation.
Materials and Methods
Preparation of Plasma-Activated Liquids (PALs)
PBS (pH 7.4) was purchased from Sigma-Aldrich (St. Louis, MO, USA) and saline (0.9% NaCl) was purchased from Hospira (Lake Forest, IL, USA). Figures 1 and 2 sketches the procedures of PAL preparation and PAL treatment of oral bacteria and mammalian cells. PBS and saline were used as the liquids in this study. 250 µl of each liquid was placed into the wells of a 48-well plate. A non-thermal atmospheric plasma brush described in our previous study [4] was inserted into each well to treat the liquids. The plasma brush was operated at 6 mA using 3000 sccm argon and 30 sccm oxygen flow rates [4]. Liquids were treated with the plasmas for 5 minutes.
Figure 1: The procedures of PAL treatment on planktonic oral bacteria or mammalian cells.
A. Bacteria Culture and Biofilm Culture [4]
A colony of S. mutans (ATCC 700610) was cultured overnight at 37°C in 5 ml TSB medium. For planktonic bacteria, the experimental bacteria suspension was obtained by diluting the overnight cultured S. mutans suspension at a ratio of 1:100 with fresh TSB medium. For biofilm application, the overnight cultured S. mutans suspension was diluted at 1:200 ratio with fresh TSB medium. 600 µl of 1:200 diluted bacterial suspensions were added to each well of a 48-well plate, and then incubated in a 37°C for 24 hours to develop biofilms on the bottoms of the wells.
B. Disinfection Against Planktonic Bacteria [17]
In order to assess the instant antibacterial effect, PALs (50 µl) was mixed with 50 µl bacterial suspension. Final bacteria density was 107 CFU/mL. The mixture was keep at room temperature for one hour, and a serial 10-fold dilution was taken. Then, 10 µl of each dilution was spread on square agar plates. The colonies were counted after 3 days.
The long-term antibacterial effect was also assessed. Another set of samples was prepared as described above. After the treatment of PAL, 100 µl fresh TSB was add to each well to provide nutrition, and the plate was incubated at 37°C for 24 hours. Finally, the recovered bacteria were quantified by MTT assay.
C. Disinfection Against S. mutans Biofilms [5]
Biofilms prepared in Section B were used to test the disinfection effect of PAL. After removing the TSB medium, 50 µl of PAL was added to each well. The well plate was placed on a shaker for one hour to let the PAL and biofilm react completely. Afterwards, the PAL was removed and the MTT assay was performed to assess PAL disinfection efficiency.
Additional sets of samples were prepared as above and used to assess the long-term effect of PAL on the biofilm. After PAL generation, 200 µl fresh TSB was add to each well, and the well plate was incubated at 37°C for 24 hours. Finally, the recovered biofilm was quantified by MTT assay.
Figure 2: The procedures of PAL treatment on S. mutanoral biofilms.
E. Toxicity Test [18] of the PALs
PALs were first prepared by exposing the liquids to plasma brush for duration ranging from 0 to 5 minutes. To assess PAL effect on mammalian cells, 150 µl of 2×104 cells/mL fibroblast cells were mixed with 50 ul PALs in the wells of a 96-well plate. The mixtures were then placed on a shaker for one hour. After incubation at 37°C for 24 hours, cell viability was assessed by MTT assay [18].
F. Chemical Property of PALs
After PALs was prepared, the H2O2 content was measured to determine its concentration in medium. The H2O2 concentrations in PALs were quantified using a Hydrogen Peroxide Assay Kit (National Diagnostics, Atlanta, GA, USA) by following the manufacturer’s instructions.
G. Statistical Analysis
SPSS statistics software (IBM, Armonk, NY) was used to perform the one-way analysis of variance (ANOVA) on all results. Turkey honestly significant difference (HSD) post hoc test was performed. If P<0.05 is detected, it is considered to have a statistically significant effect at the 95% confidence level.
Results
A. Antimicrobial Effect of PAL on Planktonic Bacteria
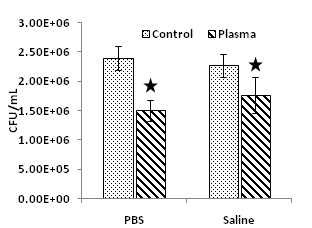
Figure 3 shows the antibacterial efficacy when planktonic S. mutans were exposed to PALs for one hour. Both PALs (PBS and saline) carried strong antimicrobial properties. As compared with the untreated controls, plasma activated PBS caused 38% bacteria reduction (P < 0.05), and plasma activated saline inactivated 22% of the bacteria (P < 0.05). Long-term antibacterial effects of PALs on planktonic S. mutans cells were also studied. As shown in figure 4, there was no significant difference (P > 0.05) on the bacterial survival percentage between the untreated liquid controls and the PALs.
Figure 3: Instant antibacterial effect of PALs on planktonic S. mutan cells after one hour mixing with PALs at room temperature. The star indicates the statistically significant difference (P < 0.05).
Figure 4: Long-term antimicrobial effect of PALs on planktonic S. mutan cells after 24 hours incubation with PALs at 37°C. No statistical difference was observed (P > 0.05).
B. Disinfection Effect of PAL against Biofilms
The disinfection efficacy of PALs against biofilm is shown in figure 5. Both PALs (PBS and saline) also carried strong antimicrobial properties against biofilms. As compared with the untreated controls, plasma activated PBS caused 25% bacterial reduction (P < 0.05), and plasma activated saline caused 18% bacterial reduction (P < 0.05). The long-term anti-bacterial effects of PALs on S. mutan biofilms were also studied. As shown in figure 6, there were no differences (P > 0.05) between the regular non-exposed biofilms and the biofilms exposed to PALs. Co-culture of the biofilms with PALs for 24 hours did not inhibit bacterial growth.
Figure 5: Instant antibacterial effect of PALs on S. mutan biofilms after one hour mixing with PALs at room temperature. The star indicates the statistically significant difference (P < 0.05).
Figure 6: Long-term antimicrobial effect of PALs on S. mutans biofilms after 24 hours incubation with PALs at 37ºC. No statistical difference was observed (P > 0.05).
C. Toxicity of PALs to Mammalian Cells Fibroblast cells were used to evaluate the toxicity of PALs against mammalian cells. Figure 7 shows the mammalian cell viability after exposure to PALs. No reduction (P>0.05) of cell viability was found with both PALs when plasma activation time changed from 0 minute to 5 minutes.
Figure 7: Toxicity test of PALs on fibroblast cells. No statistical difference was observed (P > 0.05).
D. Chemical Property of PALs
PBS and saline were plasma treated with duration ranging from 1 to 5 minutes. The H2O2 concentration in these PALs was measured. Figure 8 shows the H2O2 concentration change in PALs with plasma treatment time. The H2O2 concentration increased with plasma activation time. For PBS, the H2O2 concentration increased from 10 to 30 mg/L. For saline, H2O2 concentration increased from 25 to 41mg/L.
Figure 8: H2O2 concentration change in PALs with plasma activation time. Different notions of a and b indicate significant difference (P<0.05) exits between groups.
Discussion
The aim of this study was to investigate the disinfecting performance of PALs. PBS and saline were exposed to plasma treatment to produce PALs. The results showed that PALs could successfully inactivate bacteria in both planktonic and biofilm forms. It is known that PALs could be a broad spectrum antimicrobial agent [9]. Kamgang G, et al. provided evidence that plasma activated water could kill gram-negative and gram-positive bacteria[19]. With PALs, microorganisms were killed without being directly exposed to the plasma plume. Thus, PLAs could be used for disinfect delicate surfaces and tissues, which could be slightly damaged by direct plasma treatment [8,9]. By using PALs, tissues are not directly exposed to plasmas and thus damages resulting from the plasma treatment can be prevented. Using PALs could expand the range of potential applications for plasmas.
PAL disinfection of biofilm bacteria was found to be less efficient than planktonic bacteria. The reduced susceptibility of biofilm bacteria to various disinfection methods has been acknowledged in the literature. It was suggested that the disinfection resistance was independent of whether the bacteria were gram-negative or positive [10]. One explanation for the increased disinfection resistance is the structure and unique property of biofilm. PALs could also be regarded as a kind of antimicrobial agent [20]. Production of an exopolysaccharide matrix in biofilms could prevent the access of antimicrobials to the bacteria embedded in the biofilm community [21-24]. Furthermore, slow growth and low metabolism of bacteria in biofilms also account for the resistance of biofilm bacteria to antimicrobial agents [25].
The exact species and products generated in PALs have not yet been fully understood. More detailed chemical characterization of the PALs is needed in order to understand their antibacterial property. As reported in literature hydrogen peroxide is one of the major species generated in PALs [26]. In this study, therefore, the concentration of hydrogen peroxide in the PALs was measured. The largest amount of H2O2 was found to be 42 mg/L after 5 minutes’ plasma exposure on saline. According to literature [24], the amount of H2O2 required for inactivation was 500mg/L. This finding suggests that PALs may carry other species or products that are responsible for their antimicrobial property, which is consistent with Ercan’s study [9].
This study has limitations. First only one kind of gram-positive oral bacteria was tested. Other strains including gram-positive, gram-negative and yeast existing in oral environment should be further studied. In addition, more detailed chemical characterization of the PALs should be conducted in order to fully understand the disinfection mechanisms of PALs. The shelf life of the plasma-generated antimicrobial species in PALs is also critical. Therefore, experiments should be performed to assess the aging effects of PALs on antimicrobial efficacy.
Conclusion
The PALs showed instant antimicrobial effects on oral bacteria of S. mutans in both planktonic and biofilm forms. However, PALs could not completely disinfect all the bacteria and were not able to inhibit the bacteria recovery. It was also found that the PALs were non-toxic to mammalian cells. Plasma treatment could increase the concentration of hydrogen peroxide in the liquids, but the amount was not high enough to completely inactivate all bacteria.
Conflict of Interest
Hongmin Sun, Meng Chen and Qingsong Yu have an equity interest in Nanova, Inc.
References
- Koban I, Holtfreter B, Hübner NO, Matthes R, Sietmann R, et al. (2011). Antimicrobial efficacy of non‐thermal plasma in comparison to chlorhexidine against dental biofilms on titanium discs in vitro–proof of principle experiment. J of clin peri. 38(10):956-965.
- Xiong Z, Du T, Lu X, Cao Y, Pan Y. (2011). How deep can plasma penetrate into a biofilm? Appl Phys Lett. 98(22):221503-221503-3.
- Rupf S, Lehmann A, Hannig A, Schäfer B, Schubert A, et al. (2010). Killing of adherent oral microbes by a non-thermal atmospheric plasma jet. J of medi microbi. 59(2):206-212.
- Hong Q, Dong X, Chen M, Xu Y, Sun H, et al. (2016). Disinfection of Streptococcus mutans biofilm by a non-thermal atmospheric plasma brush. Japanese J of Appl Phys. 55(7S2):07LG02.
- Hong Q, Dong X, Chen M, Sun H, Hong L, et al. (2019). An in vitro and in vivo study of plasma treatment effects on oral biofilms. Journal of Oral Microbiology. 11(1):1603524.
- Sladek R, Filoche S, Sissons C, Stoffels E. (2007). Treatment of Streptococcus mutans biofilms with a nonthermal atmospheric plasma. Lett in Appl Micr. 45(3):318-323.
- Koban I, Matthes R, Hübner NO, Welk A, Meisel P, et al. (2010). Treatment of Candida albicans biofilms with low-temperature plasma induced by dielectric barrier discharge and atmospheric pressure plasma jet. New J of Phys. 12(7):073039.
- Joshi SG, Yost A, Joshi SS, Addya S, Ehrlich G, et al. (2015). Microarray Analysis of Transcriptomic Response of Escherichia coli to Nonthermal Plasma-Treated PBS Solution. Adv in Biosand Biot. 06(02):14.
- Ercan UK, Wang H, Ji H, Fridman G, Brooks A, et al. (2013). Nonequilibrium Plasma-Activated Antimicrobial Solutions are Broad-Spectrum and Retain their Efficacies for Extended Period of Time. Plas Proc and Pol. 10(6):544-555.
- Kamgang-Youbi G, Herry JM, Meylheuc T, Brisset JL, Bellon-Fontaine MN, et al. (2009). Microbial inactivation using plasma-activated water obtained by gliding electric discharges. Lett in Appl Micr. 48(1):13-18.
- Du CM, Wang J, Zhang L, Li HX, Liu H, et al. (2012). Corrigendum: The application of a non-thermal plasma generated by gas–liquid gliding arc discharge in sterilization. New J of Phys. 14(10):109502.
- Mohades S. Laroussi M, Sears J, Barekzi N, Razavi H. (2015). Evaluation of the effects of a plasma activated medium on cancer cells. Phys of Plas. 22(12):122001.
- Bruggeman PJ, Kushner MJ, Locke BR, Gardeniers JGE, Graham WG, et al. (2016). Plasma–liquid interactions: a review and roadmap. Plas Sour Sci and Tech. 25(5):053002.
- Lukes P, Dolezalova E, SisrovaI, Clupek M. (2014). Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H 2 O 2 and HNO 2. Plas Sour Sci and Tech. 23(1):015019.
- Lukes P, Brisset JL, Locke BR. (2012). Biological Effects of Electrical Discharge Plasma in Water and in Gas–Liquid Environments, in Plasma Chemistry and Catalysis in Gases and Liquids. Wiley-VCH Verlag GmbH & Co. KGaA. p. 309-352.
- Oehmige, Winter J, Hähnel M, Wilke C, Brandenburg R, et al. (2011). Estimation of Possible Mechanisms of Escherichia coli Inactivation by Plasma Treated Sodium Chloride Solution. Plas Proc and Pol. 8(10):904-913.
- Yang B, Chen J, Yu Q, Li h, Lin M, et al. (2011). Oral bacterial deactivation using a low-temperature atmospheric argon plasma brush. J of dent. 39(1):48-56.
- García JL, Asadinezhad A, Pacherník J, Lehocký M, Junkar I, et al. (2010). Cell Proliferation of HaCaT Keratinocytes on Collagen Films Modified by Argon Plasma Treatment. Molecules. 15(4):2845.
- Kamgang-Youbi G, Herry JM, Bellon-Fontaine MN, Brisset JL, Doubla A, et al. (2007). Evidence of Temporal Postdischarge Decontamination of Bacteria by Gliding Electric Discharges: Application to Hafnia alvei. Appl and Env Micr. 73(15):4791-4796.
- Traylor MJ, Pavlovich MJ, Karim S, Hait P, Sakiyama Y, et al. (2011). Long-term antibacterial efficacy of air plasma-activated water. J of Phys D: Appl Phys. 44(47):472001.
- Marsh PD. (2005). Dental plaque: biological significance of a biofilm and community life-style. J of clin peri. 32:7-15.
- Marsh PD. (2010). Controlling the oral biofilm with antimicrobials. J of dent. 38, Supplement. 1(0):S11-S15.
- Xu KD, McFeters GA, Stewart PS. (2000). Biofilm resistance to antimicrobial agents. Micr. 146(3):547-549.
- Stewart PS. (1996). Theoretical aspects of antibiotic diffusion into microbial biofilms. Anti Agen and Chem. 40(11):2517-2522.
- MahTFC, O'Toole GA. (2001). Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 9(1):34-39.
- Joshi SG, Cooper M, Yost A, Paff M, Ercan UK, Friedman G, et al. (2011). Nonthermal Dielectric-Barrier Discharge Plasma-Induced Inactivation Involves Oxidative DNA Damage and Membrane Lipid Peroxidation in Escherichia coli. Anti Agen and Chem. 55(3):1053-1062.
Copyrights: Yu Q, et al. (2021). This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Yu Q, et al. (2021). The Antimicrobial Property of Plasma Activated Liquids (PALs) against Oral Bacteria Streptococcus mutans. Dental. 3(1):07.
 Abstract
Abstract  PDF
PDF


.jpg)


